Contents
- 1 Introduction
- 2 Physisorption
- 3 Chemisorption
- 4 Differences between Physisorption and Chemisorption
- 5 Applications of physisorption:
- 6 Applications of chemisorption:
- 7 Summary
- 8 FAQs
- 8.1 What is Physisorption?
- 8.2 What is Chemisorption?
- 8.3 What is the main difference between Physisorption and Chemisorption?
- 8.4 What type of molecules are typically involved in Physisorption?
- 8.5 What type of molecules are typically involved in Chemisorption?
- 8.6 How is the adsorption energy different in Physisorption and Chemisorption?
- 8.7 Can Physisorption and Chemisorption occur simultaneously?
- 8.8 What are some applications of Physisorption and Chemisorption?
Learn about the key differences between physisorption and chemisorption, two important types of adsorption. Discover the types of molecules involved, the strength of the interactions, and the applications of each process.
Introduction
Adsorption is a widely used process in various fields such as gas separation, catalysis, drug delivery, and surface modification. Two types of adsorption, physisorption and chemisorption, are characterized based on the nature of the bonding forces between the adsorbate and adsorbent. Understanding the differences between physisorption and chemisorption is crucial for the development of advanced materials and the optimization of industrial processes. In this blog, we will discuss the differences between physisorption and chemisorption, their characteristics, examples, and applications.
Physisorption
Physisorption is a type of adsorption in which the adsorbate molecules are attracted to the surface of the adsorbent through weak van der Waals forces or electrostatic interactions. The bonding forces in physisorption are relatively weak, and the adsorption is generally reversible. Physisorption occurs at low temperatures and can be characterized by the adsorption isotherms that exhibit a plateau at higher pressures.
Examples of physisorption include nitrogen adsorption on activated carbon and water adsorption on silica gel. Physisorption is widely used in gas separation processes, where the adsorbent selectively adsorbs one gas molecule over another based on the difference in their adsorption energies. Physisorption is also used in catalysis and drug delivery, where the adsorbent acts as a carrier for the reactants or drugs.
Chemisorption
Chemisorption is a type of adsorption in which the adsorbate molecules are chemically bonded to the surface of the adsorbent. The bonding forces in chemisorption are stronger than those in physisorption and are typically characterized by covalent or ionic bonds. Chemisorption is generally irreversible and occurs at high temperatures.
Examples of chemisorption include the adsorption of hydrogen and oxygen on metal surfaces. Chemisorption is widely used in catalysis, where the adsorbate molecules are activated by the surface atoms of the adsorbent, leading to a chemical reaction. Chemisorption is also used for surface modification of materials, where the adsorbate molecules react with the surface of the adsorbent to form a desired surface layer.
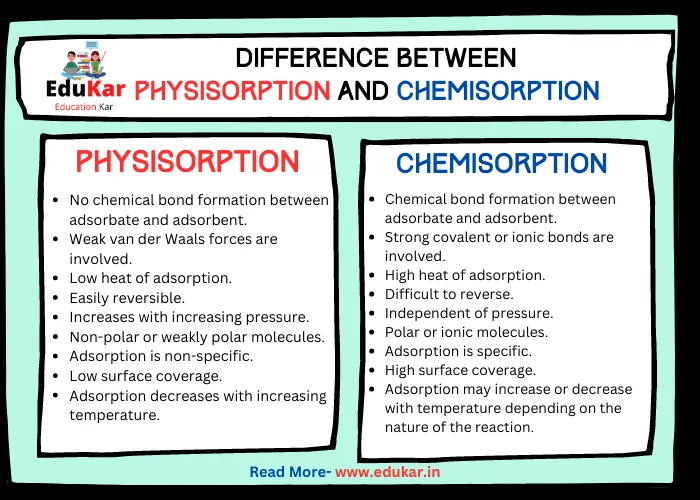
Differences between Physisorption and Chemisorption
| Property | Physisorption | Chemisorption |
|---|---|---|
| Bond Formation | No chemical bond formation between adsorbate and adsorbent | Chemical bond formation between adsorbate and adsorbent |
| Energy Involved | Weak van der Waals forces are involved | Strong covalent or ionic bonds are involved |
| Heat of Adsorption | Low heat of adsorption | High heat of adsorption |
| Reversibility | Easily reversible | Difficult to reverse |
| Adsorption at Low Pressure | Increases with increasing pressure | Independent of pressure |
| Nature of Adsorbate | Non-polar or weakly polar molecules | Polar or ionic molecules |
| Specificity | Adsorption is non-specific | Adsorption is specific |
| Surface Coverage | Low surface coverage | High surface coverage |
| Temperature Effect | Adsorption decreases with increasing temperature | Adsorption may increase or decrease with temperature depending on the nature of the reaction |
Applications of physisorption:
1. Drug delivery: Physisorption is used in drug delivery, where drugs are adsorbed onto the surface of the adsorbent, leading to controlled release of the drug. In this process, the adsorbent can be designed to have a specific release rate and duration, which can improve the efficacy and safety of the drug.
2. Environmental remediation: Physisorption is used in environmental remediation, where pollutants are removed from the environment. In this process, the adsorbate molecules are adsorbed onto the surface of the adsorbent, leading to a decrease in the concentration of the pollutant in the environment. Physisorption is used in various types of environmental remediation, such as water treatment, air purification, and soil remediation.
3. Energy storage: Physisorption is used in energy storage, where gases are stored in porous materials. In this process, the adsorbate molecules are adsorbed onto the surface of the adsorbent, leading to a high storage capacity of the gas. Physisorption is used in various types of energy storage, such as hydrogen storage, natural gas storage, and carbon dioxide storage.
4. Surface area measurement: Physisorption is used to measure the surface area of materials. In this process, a known amount of gas is adsorbed onto the surface of the adsorbent, and the amount of gas adsorbed is measured. The amount of gas adsorbed can be used to calculate the surface area of the adsorbent. Surface area measurement is used in various industries, such as materials science, chemical engineering, and pharmaceuticals.
Applications of chemisorption:
Chemisorption has numerous applications in various fields. Some of the major applications of chemisorption are:
1. Catalysis: Chemisorption is widely used in catalysis. In this process, the adsorbate molecules are activated by the surface atoms of the adsorbent, leading to a chemical reaction. The chemisorption process can increase the reaction rate, selectivity, and efficiency of the catalytic reaction. Chemisorption is used in various types of catalysis, such as heterogeneous catalysis, homogeneous catalysis, and enzyme catalysis.
2. Surface modification of materials: Chemisorption is used for surface modification of materials, where the adsorbate molecules react with the surface of the adsorbent to form a desired surface layer. This process can improve the surface properties of the material, such as corrosion resistance, adhesion, and biocompatibility. Chemisorption is used in various industries, such as automotive, aerospace, and biomedical.
3. Gas sensors: Chemisorption is used in gas sensors for the detection of gases. In this process, the adsorbate molecules react with the surface of the adsorbent, leading to a change in the electrical properties of the adsorbent. The change in the electrical properties can be measured and used to detect the presence of the gas.
4. Adsorption refrigeration: Chemisorption is used in adsorption refrigeration, which is an environmentally friendly and energy-efficient cooling technology. In this process, the adsorbate molecules are adsorbed onto the surface of the adsorbent, leading to a decrease in the temperature of the system. This process can be used for air conditioning, refrigeration, and cooling in various industries.
5. Chemical sensing: Chemisorption is used in chemical sensing for the detection of chemicals. In this process, the adsorbate molecules react with the surface of the adsorbent, leading to a change in the optical or electrical properties of the adsorbent. The change in the properties can be measured and used to detect the presence of the chemical. Chemisorption-based chemical sensors are used in various applications, such as environmental monitoring, food safety, and medical diagnosis.
Summary
Physisorption and chemisorption are two important types of adsorption that are characterized by different bonding forces, reversibility, temperature dependence, and adsorption mechanisms. Understanding the differences between physisorption and chemisorption is crucial for the development of advanced materials and the optimization of industrial processes. Physisorption is used in gas separation, catalysis, and drug delivery, while chemisorption is used for catalysis and surface modification of materials. Future prospects in the field include the development of new adsorbent materials with enhanced selectivity and stability, as well as the optimization of existing industrial processes to reduce energy consumption and increase efficiency.
FAQs
What is Physisorption?
Physisorption is a type of adsorption where molecules are attracted to the surface of a material through weak Van der Waals forces. The adsorbent and the adsorbate do not form any chemical bonds.
What is Chemisorption?
Chemisorption is a type of adsorption where a chemical reaction occurs between the adsorbate and the surface of the adsorbent. The adsorbent and the adsorbate form a chemical bond.
What is the main difference between Physisorption and Chemisorption?
The main difference between Physisorption and Chemisorption is the strength of the interaction between the adsorbate and the adsorbent. In Physisorption, the interaction is weak and physical, whereas in Chemisorption, it is strong and chemical.
What type of molecules are typically involved in Physisorption?
Physisorption is typically observed for non-polar or weakly polar molecules, such as noble gases, hydrocarbons, and water.
What type of molecules are typically involved in Chemisorption?
Chemisorption is typically observed for molecules with reactive functional groups, such as alcohols, amines, and carboxylic acids.
How is the adsorption energy different in Physisorption and Chemisorption?
The adsorption energy in Chemisorption is much higher than in Physisorption because a chemical bond is formed between the adsorbate and the adsorbent.
Can Physisorption and Chemisorption occur simultaneously?
Yes, Physisorption and Chemisorption can occur simultaneously, especially when the adsorbate molecule has both polar and nonpolar groups. In this case, the polar groups may chemisorb onto the surface while the nonpolar groups physisorb.
What are some applications of Physisorption and Chemisorption?
Physisorption is used in gas storage, separation, and purification, while Chemisorption is used in catalysis, chemical sensors, and drug delivery.