Contents
- 1 Introduction
- 2 Order of Reaction
- 3 Molecularity of Reaction
- 4 Differences between Order and Molecularity
- 5 Conclusion
- 6 FAQs related to Order and Molecularity for Exams
- 6.1 What is the difference between order and molecularity in a chemical reaction?
- 6.2 How is the order of a chemical reaction determined?
- 6.3 What is molecularity in a chemical reaction?
- 6.4 What is the relationship between order and molecularity?
- 6.5 Can a reaction have different orders with respect to different reactants?
Learn about the difference between order and molecularity in a chemical reaction. Find out how to determine the order of a reaction and what molecularity means. Discover the relationship between order and molecularity and whether a reaction can have different orders with respect to different reactants.
Introduction
Chemical reactions are fundamental processes that occur in both living and non-living systems. They involve the transformation of one or more substances into new substances, accompanied by the release or absorption of energy. Understanding the kinetics of chemical reactions is essential in predicting and controlling the rate and direction of reactions. Two important concepts in chemical kinetics are the order of reaction and the molecularity of reaction.
Order of Reaction
The order of a reaction is the power to which the concentration of a reactant is raised in the rate equation. In other words, it describes how the rate of the reaction is affected by changes in the concentration of a single reactant. The order of a reaction can be zero, first, second, or higher. The order of a reaction can be determined experimentally by measuring the initial rates of reaction at different concentrations of the reactants.
For example, if doubling the concentration of reactant A doubles the rate of reaction, the reaction is first-order with respect to A.
Molecularity of Reaction
The molecularity of a reaction is the number of molecules or ions that participate in the elementary reaction step. It describes the number of reactant species that collide to form products in a single step. The molecularity of a reaction can be unimolecular, bimolecular, or trimolecular. Unimolecular reactions involve a single reactant molecule that decomposes into one or more products. Bimolecular reactions involve two reactant molecules that collide to form a product. Trimolecular reactions involve three reactant molecules that collide to form a product.
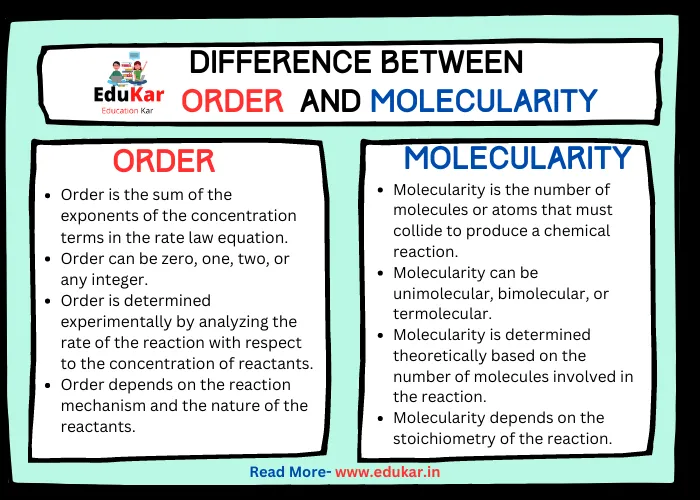
Differences between Order and Molecularity
| S.No, | Order | Molecularity |
|---|---|---|
| 1 | Order is the sum of the exponents of the concentration terms in the rate law equation. | Molecularity is the number of molecules or atoms that must collide to produce a chemical reaction. |
| 2 | Order can be zero, one, two, or any integer. | Molecularity can be unimolecular, bimolecular, or termolecular. |
| 3 | Order is determined experimentally by analyzing the rate of the reaction with respect to the concentration of reactants. | Molecularity is determined theoretically based on the number of molecules involved in the reaction. |
| 4 | Order depends on the reaction mechanism and the nature of the reactants. | Molecularity depends on the stoichiometry of the reaction. |
| 5 | Order can be fractional or negative in some cases. | Molecularity is always a whole number. |
| 6 | Order can change as the reaction progresses or under different reaction conditions. | Molecularity is constant for a given reaction. |
| 7 | Order can be used to predict the effect of changes in concentration on the rate of the reaction. | Molecularity is not used to predict the effect of changes in concentration on the rate of the reaction. |
| 8 | Order is used to describe the reaction kinetics. | Molecularity is used to describe the reaction mechanism. |
| 9 | Order is a property of the rate law equation. | Molecularity is a property of the reaction itself. |
| 10 | Order is related to the rate constant of the reaction. | Molecularity is not related to the rate constant of the reaction. |
| 11 | Order is independent of the stoichiometry of the reaction. | Molecularity depends on the stoichiometry of the reaction. |
| 12 | Order can be different for the same reaction under different conditions. | Molecularity is always the same for a given reaction. |
| 13 | Order is useful for comparing the rates of different reactions. | Molecularity is not useful for comparing the rates of different reactions. |
| 14 | Order is a more commonly used concept in chemical kinetics. | Molecularity is a less commonly used concept in chemical kinetics. |
| 15 | Order is affected by temperature, pressure, and catalysts. | Molecularity is not affected by temperature, pressure, and catalysts. |
The order of a reaction and the molecularity of a reaction are two distinct concepts in chemical kinetics. The order of a reaction describes the concentration dependence of the rate of reaction, while the molecularity of a reaction describes the number of reactant species involved in a single elementary reaction step. While these two concepts are related, they are not interchangeable. A reaction can be first-order with respect to one reactant and bimolecular with respect to another reactant.
For example, consider the reaction A + 2B → C. The order of the reaction with respect to A is first-order because doubling the concentration of A doubles the rate of reaction. The order of the reaction with respect to B is second-order because the rate of reaction is proportional to the square of the concentration of B. The molecularity of the reaction is bimolecular because two reactant species (A and B) collide to form a product.
Conclusion
The order of a reaction and the molecularity of a reaction are two critical concepts in chemical kinetics. The order of a reaction describes the concentration dependence of the rate of reaction, while the molecularity of a reaction describes the number of reactant species involved in a single elementary reaction step. The order of a reaction and the molecularity of a reaction are related but distinct concepts. Understanding the difference between order and molecularity is crucial in predicting and controlling the rate and direction of chemical reactions. By determining the order of a reaction and the molecularity of a reaction, chemists can gain insights into the reaction mechanism and optimize reaction conditions to achieve desired outcomes.
What is the difference between order and molecularity in a chemical reaction?
Order and molecularity are two different concepts that describe different aspects of a chemical reaction. Order refers to the dependence of the reaction rate on the concentration of one reactant or several reactants, while molecularity refers to the number of molecules or particles involved in the rate-determining step of the reaction.
How is the order of a chemical reaction determined?
The order of a chemical reaction can be determined experimentally by measuring the reaction rate at different concentrations of the reactants. If the reaction rate is proportional to the concentration of one reactant, the reaction is said to be first-order with respect to that reactant. If the reaction rate is proportional to the concentration of two reactants, the reaction is said to be second-order with respect to those reactants. If the reaction rate is independent of the concentration of the reactants, the reaction is said to be zero-order.
What is molecularity in a chemical reaction?
Molecularity refers to the number of molecules or particles that participate in the rate-determining step of a chemical reaction. For example, a unimolecular reaction involves only one molecule in the rate-determining step, while a bimolecular reaction involves two molecules. The molecularity of a reaction is determined by its reaction mechanism, which describes the sequence of steps that lead from reactants to products.
What is the relationship between order and molecularity?
The order of a chemical reaction and its molecularity are two different concepts that describe different aspects of the reaction. While the order of a reaction depends on the concentration of the reactants, the molecularity of a reaction is determined by its reaction mechanism. In general, the order of a reaction is not necessarily equal to its molecularity, but the molecularity can provide insight into the order of the reaction.
Can a reaction have different orders with respect to different reactants?
Yes, a reaction can have different orders with respect to different reactants. For example, a reaction could be first-order with respect to one reactant and second-order with respect to another reactant. The overall order of the reaction would then be the sum of the individual orders with respect to each reactant.