Learn the key differences between soap and detergent in this informative article by Edukar. Discover how these two cleaning agents differ in terms of ingredients, properties, and environmental impact.
Soap and detergent are common household items that are essential for daily cleaning tasks. While both of these products are designed to clean, they are different in composition, properties, and applications.
Definition of Soap and Detergent
Soap and detergent are cleaning agents that help to remove dirt, grime, and stains from surfaces. Soap is a product made from natural ingredients such as oils, fats, and alkali. Detergent is a synthetic product made from petrochemicals or oleochemicals.
Composition of Soap and Detergent
Soap is made up of natural ingredients that are biodegradable, such as animal fats or vegetable oils, and alkali. The alkali reacts with the fats or oils in a process called saponification, producing soap and glycerin. The soap molecules have two parts, one end that is hydrophilic (attracted to water) and the other end that is hydrophobic (repelled by water).
Detergent, on the other hand, is made up of synthetic ingredients that are not biodegradable. These ingredients are usually derived from petrochemicals or oleochemicals, and they are combined with various additives to produce the desired cleaning properties.
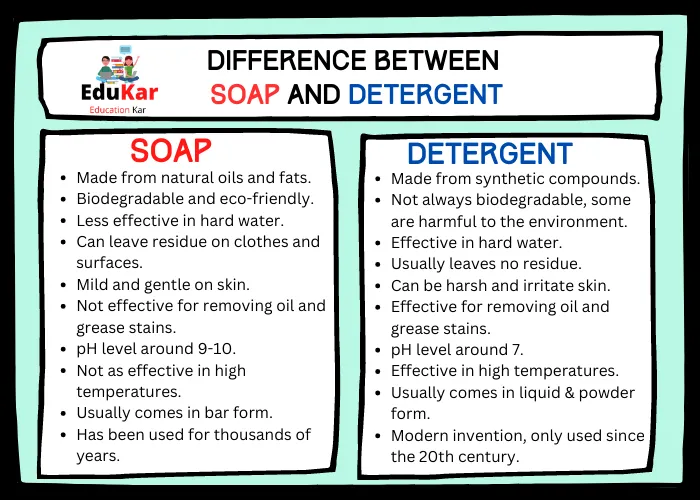
Difference Between Soap and Detergent
| Soap | Detergent |
|---|---|
| Made from natural oils and fats | Made from synthetic compounds |
| Biodegradable and eco-friendly | Not always biodegradable, some are harmful to the environment |
| Less effective in hard water | Effective in hard water |
| Can leave residue on clothes and surfaces | Usually leaves no residue |
| Mild and gentle on skin | Can be harsh and irritate skin |
| Not effective for removing oil and grease stains | Effective for removing oil and grease stains |
| pH level around 9-10 | pH level around 7 |
| Not as effective in high temperatures | Effective in high temperatures |
| Usually comes in bar form | Usually comes in liquid form |
| Has been used for thousands of years | Modern invention, only used since the 20th century |
Properties of Soap and Detergent
Cleaning ability is one of the most important properties of soap and detergent. Soap is good at cleaning organic materials, such as oils, fats, and proteins. However, it is not very effective in hard water, which contains high levels of minerals. Detergent, on the other hand, is effective in both soft and hard water, making it a better choice for many cleaning tasks.
The pH level of soap and detergent is also different. Soap is alkaline, with a pH level of around 9-10, while detergent is neutral or slightly acidic, with a pH level of around 7-8. This difference in pH level can affect how effective the product is at cleaning, as well as its impact on the environment.
Another important property to consider is the effect on the environment. Soap is made from natural ingredients that are biodegradable, making it a more environmentally friendly choice. Detergent, on the other hand, is made from synthetic ingredients that are not biodegradable and can harm aquatic life if not disposed of properly.
Applications of Soap and Detergent
Soap has been used for centuries for cleaning purposes, and it is still a popular choice for many tasks today. Some common uses of soap include washing hands and body, cleaning clothes, and washing dishes. Soap is also used in many personal care products, such as shampoo and body wash.
Detergent is also widely used for cleaning tasks, especially in household cleaning products, such as laundry detergent, dish soap, and all-purpose cleaners. Detergent is often the preferred choice for cleaning clothes, as it is more effective in hard water and can remove stains more easily than soap.
Safety and Health Considerations
Both soap and detergent can be hazardous if not used properly. Soap can cause skin irritation or allergic reactions in some people, especially if it contains fragrances or other additives. Detergent can also cause skin irritation or allergic reactions, as well as eye irritation and respiratory problems if inhaled.
To avoid these risks, it’s important to take precautions when using soap or detergent. Always follow the instructions on the label, wear gloves when necessary, and avoid inhaling or ingesting the product.