Learn about the difference between roasting and calcination. This informative article explains the distinctions between these two common high-temperature processes, including their purposes and the chemical reactions involved. Whether you’re a student of chemistry or just curious about these industrial techniques, this article is sure to enlighten and educate.
Introduction
Roasting and calcination are two thermal processing techniques that have been used for centuries to produce a wide range of materials. These processes involve heating materials to high temperatures, but they differ in their purpose and the conditions required for their execution. Understanding the differences between these two processes is crucial in determining which technique to use in various industries. In this blog, we will explore the differences between roasting and calcination in detail.
Roasting
Roasting is a thermal process that involves heating a material in the presence of oxygen. The process is commonly used to extract metals from ores or to drive off impurities in a material. Some examples of roasting processes include the roasting of sulfide ores to produce metal oxides and the roasting of coffee beans to produce the desired flavor and aroma.
The conditions required for roasting include high temperature and the presence of oxygen. The temperature range for roasting is typically between 500 and 1000°C. Roasting can also occur at atmospheric pressure, but some roasting processes may require high pressure.
The purpose of roasting is to drive off impurities from the material being heated, change the physical or chemical properties of the material, or to produce a product with a desired color, flavor, or aroma. Roasting differs from calcination in that it requires the presence of oxygen and is used primarily in metallurgy and the food industry.
Calcination
Calcination is a thermal process that involves heating a material in the absence of oxygen. The process is used to drive off volatile components, decompose materials, or to produce stable compounds. Some examples of calcination processes include the calcination of limestone to produce lime and the calcination of gypsum to produce plaster of Paris.
The conditions required for calcination include high temperature and the absence of oxygen. The temperature range for calcination is typically between 600 and 1000°C. Calcination may occur at atmospheric pressure, but some processes may require high pressure.
The purpose of calcination is to drive off volatile components from the material being heated, decompose materials, or produce stable compounds. Calcination differs from roasting in that it occurs in the absence of oxygen and is used primarily in the chemical industry.
Difference between Roasting and Calcination
| Roasting | Calcination | |
|---|---|---|
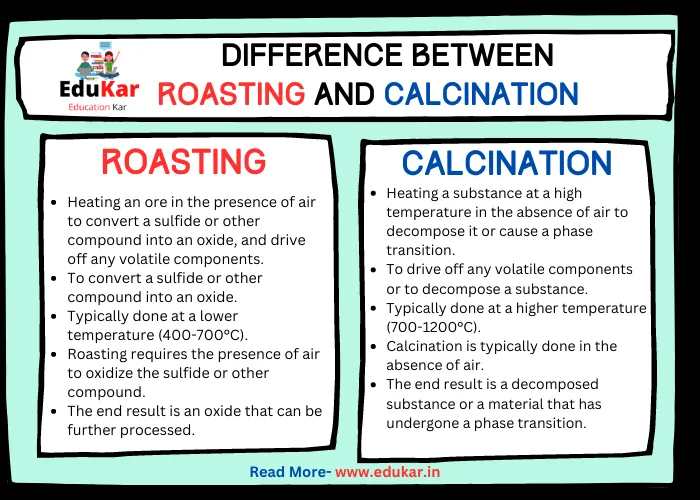
| Definition | Heating an ore in the presence of air to convert a sulfide or other compound into an oxide, and drive off any volatile components | Heating a substance at a high temperature in the absence of air to decompose it or cause a phase transition |
| Purpose | To convert a sulfide or other compound into an oxide | To drive off any volatile components or to decompose a substance |
| Temperature | Typically done at a lower temperature (400-700°C) | Typically done at a higher temperature (700-1200°C) |
| Presence of Air | Roasting requires the presence of air to oxidize the sulfide or other compound | Calcination is typically done in the absence of air |
| End Result | The end result is an oxide that can be further processed | The end result is a decomposed substance or a material that has undergone a phase transition |
| Examples | Roasting is commonly used to extract metals from sulfide ores, such as copper, lead, and zinc | Calcination is commonly used to create cement from limestone, or to produce quicklime from calcium carbonate |
| Chemical Changes | During roasting, there is a chemical change where the sulfide or other compound is oxidized | During calcination, there is a chemical change where the substance is decomposed or undergoes a phase transition |
| Process Time | Roasting can take a shorter amount of time compared to calcination | Calcination may take a longer amount of time compared to roasting |
| Equipment | Roasting can be done in furnaces, kilns, or roasters | Calcination is typically done in specialized equipment such as rotary kilns or fluidized bed reactors |
| Industrial Applications | Roasting is widely used in the metallurgical industry | Calcination is widely used in industries such as cement production, lime production, and ceramics |
Applications of Roasting
Metallurgy: Roasting is commonly used in metallurgy to extract metals from ores. The process involves heating the ore in the presence of oxygen to convert the metal sulfides into metal oxides. This process is known as oxidation roasting and is used to extract metals such as copper, zinc, and lead.
Food Industry: Roasting is commonly used in the food industry to improve the flavor, aroma, and appearance of food products. Examples of food products that are roasted include coffee beans, cocoa beans, and nuts.
Environmental: Remediation Roasting can also be used for environmental remediation, such as the removal of organic compounds from contaminated soils. The process involves heating the soil in the presence of oxygen to decompose the organic compounds.
Applications of Calcination
Chemical Industry: Calcination is commonly used in the chemical industry to produce various chemicals, such as lime, cement, and plaster of Paris. The process involves heating a material in the absence of oxygen to drive off volatile components, decompose materials, or produce stable compounds.
Catalyst Production: Calcination is also used in the production of catalysts. The process involves heating a mixture of metal oxides and other materials in the absence of oxygen to produce a stable compound that can be used as a catalyst.
Ceramics Industry: Calcination is used in the ceramics industry to produce ceramic powders. The process involves heating a mixture of metal oxides in the absence of oxygen to produce a fine powder that can be used to make ceramic products.
Conclusion
Roasting and calcination are two thermal processing techniques used to produce a wide range of materials. Roasting involves heating a material in the presence of oxygen and is used primarily in the food and metallurgy industries. Calcination involves heating a material in the absence of oxygen and is used primarily in the chemical industry. Understanding the differences between these two processes is crucial in determining which technique to use in various industries.
FAQs
What is roasting?
Roasting is a process of heating an ore in the presence of air to convert a sulfide or other compound into an oxide and drive off any volatile components.
What is calcination?
Calcination is a process of heating a substance at a high temperature in the absence of air to decompose it or cause a phase transition.
What is the purpose of roasting?
The purpose of roasting is to convert a sulfide or other compound into an oxide that can be further processed
What is the purpose of calcination?
The purpose of calcination is to drive off any volatile components or to decompose a substance.
What is the temperature range for roasting?
Roasting is typically done at a lower temperature range of 400-700°C.
What is the temperature range for calcination?
Calcination is typically done at a higher temperature range of 700-1200°C.
Does roasting require the presence of air?
Yes, roasting requires the presence of air to oxidize the sulfide or other compound.
Is calcination typically done in the absence of air?
Yes, calcination is typically done in the absence of air.
What are some examples of roasting applications?
Roasting is commonly used to extract metals from sulfide ores, such as copper, lead, and zinc.
What are some examples of calcination applications?
Calcination is commonly used to create cement from limestone, or to produce quicklime from calcium carbonate.