Looking to understand the differences between displacement and double displacement reactions? Our guide covers everything you need to know, from the definition and characteristics of each type of reaction to their applications in biology and industry.
Introduction
Chemical reactions are the fundamental processes that drive all natural phenomena. Understanding these processes is crucial in many fields such as medicine, engineering, and agriculture. Chemical reactions can be classified into different categories, based on their characteristics and mechanisms. Two common types of chemical reactions are displacement reactions and double displacement reactions.
Displacement Reactions
A displacement reaction is a type of chemical reaction in which an element in a compound is replaced by another element. This type of reaction usually occurs when a more reactive element displaces a less reactive element from its compound.
Characteristics of Displacement Reactions
Displacement reactions have some specific characteristics. These include:
- The exchange of ions or atoms between reactants.
- Occur when a more reactive element displaces a less reactive element from its compound.
- Involves the transfer of electrons between the reactants.
Examples of Displacement Reactions
Displacement reactions can occur in a variety of situations. Here are some examples of displacement reactions:
- Zinc displacing copper in copper sulfate solution: When a piece of zinc metal is placed in a solution of copper sulfate, the zinc will displace the copper from the compound, and copper metal will be formed.
- Iron displacing copper in copper sulfate solution: If a piece of iron is placed in a solution of copper sulfate, the iron will displace the copper from the compound, and copper metal will be formed.
Double Displacement Reactions
Double displacement reactions are a type of chemical reaction that occurs when two ionic compounds are mixed, and the ions switch partners to form two new compounds. In this type of reaction, the cations and anions of the two reactants exchange places.
Characteristics of Double Displacement Reactions
Double displacement reactions have some specific characteristics. These include:
- Involves the exchange of ions between two reactants.
- Occurs when two ionic compounds are mixed.
- The ions switch partners to form two new compounds.
Examples of Double Displacement Reactions
Double displacement reactions can occur in a variety of situations. Here are some examples of double displacement reactions:
- Sodium chloride and silver nitrate forming sodium nitrate and silver chloride: When sodium chloride and silver nitrate are mixed, the sodium ions and nitrate ions combine to form sodium nitrate, while the silver ions and chloride ions combine to form silver chloride.
- Barium chloride and sulfuric acid forming barium sulfate and hydrochloric acid: When barium chloride and sulfuric acid are mixed, the barium ions combine with the sulfate ions to form barium sulfate, while the hydrogen ions combine with the chloride ions to form hydrochloric acid.
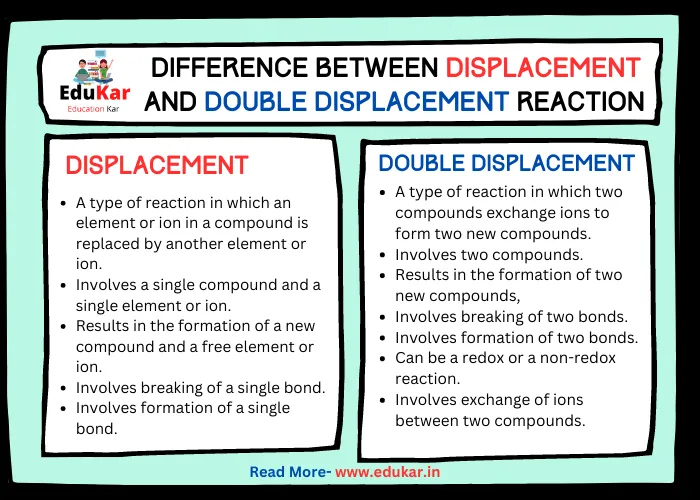
Difference between Displacement and Double Displacement Reaction
| Displacement Reaction | Double Displacement Reaction | |
|---|---|---|
| Definition | A type of reaction in which an element or ion in a compound is replaced by another element or ion | A type of reaction in which two compounds exchange ions to form two new compounds |
| Type of Reactants | Involves a single compound and a single element or ion | Involves two compounds |
| Type of Products | Results in the formation of a new compound and a free element or ion | Results in the formation of two new compounds |
| Type of Bonds Broken | Involves breaking of a single bond | Involves breaking of two bonds |
| Type of Bonds Formed | Involves formation of a single bond | Involves formation of two bonds |
| Redox Reaction | Always involves a redox reaction | Can be a redox or a non-redox reaction |
| Oxidation-Reduction | Involves a transfer of electrons from one element to another | Involves exchange of ions between two compounds |
| Precipitation | Does not always result in precipitation | Often results in precipitation |
| Acid-Base Reaction | Does not involve acid-base reactions | Can involve acid-base reactions |
| Examples | Zinc displacing copper from copper sulphate solution | Sodium chloride reacting with silver nitrate to form silver chloride and sodium nitrate |
| Chemical Equation | A + BC → AC + B | AB + CD → AD + CB |
| Common in | Single replacement reactions in general chemistry | Double replacement reactions in inorganic chemistry |
| Stoichiometry | Usually involves stoichiometric ratios between reactants and products | May involve stoichiometric or non-stoichiometric ratios between reactants and products |
| Energy Change | May involve exothermic or endothermic reactions | May involve exothermic or endothermic reactions |
| State Symbols | State symbols of reactants and products are usually included | State symbols of reactants and products are usually included |
Conclusion
Displacement and double displacement reactions are two types of chemical reactions that differ in their mechanisms and characteristics. Displacement reactions involve the exchange of ions or atoms between reactants, occur when a more reactive element displaces a less reactive element from its compound, and result in the formation of an element and a compound. Double displacement reactions involve the exchange of ions between two reactants, occur when two ionic compounds are mixed, and result in the formation of two new compounds. Understanding the differences between these two types of reactions is important in many fields, such as chemistry, biology, and physics, as it helps to predict and understand the behavior of chemicals in different environments.
FAQs:
What is a displacement reaction?
A displacement reaction is a type of chemical reaction in which an element in a compound is replaced by another element. This usually occurs when a more reactive element displaces a less reactive element from its compound.
What is a double displacement reaction?
A double displacement reaction is a type of chemical reaction that occurs when two ionic compounds are mixed, and the ions switch partners to form two new compounds.
How do displacement and double displacement reactions differ?
Displacement reactions involve the exchange of ions or atoms between reactants, occur when a more reactive element displaces a less reactive element from its compound, and result in the formation of an element and a compound. Double displacement reactions involve the exchange of ions between two reactants, occur when two ionic compounds are mixed, and result in the formation of two new compounds.
Can displacement and double displacement reactions occur in biological systems?
Yes, displacement and double displacement reactions occur in biological systems. For example, enzymes in the body catalyze these types of reactions to perform vital functions.
Can displacement and double displacement reactions be used in industry?
Yes, both types of reactions have industrial applications. For example, double displacement reactions can be used in water treatment to remove impurities, and displacement reactions can be used in the production of metals.